Part:BBa_K2675054
sfGFP-LVAtag expression cassette under the control of pAimX(full-2)-J23110 hybrid promoter
This part is an sfGFP-LVAtag (BBa_K2675006) expression cassette under the control of pAimX(full-2)-J23110 hybrid promoter (BBa_K2675024).
Usage and Biology
The switch from lytic-to-lysogenic cycle of the Bacillus phage phi3T is based on the expression of a single transcript, AimX [1]. The expression of AimX is controlled by the transcriptional regulator AimR (BBa_K2279000) which is inactivated upon binding of the ‘arbitrium’ hexapeptide SAIRGA.
The transcription of AimX is driven from the intergenic region between AimP and AimX which we will refer to as pAimX(full) promoter (BBa_K2675020). This corresponds to nucleotides 70182 to 70313 of Phi3T phage genome (GenBank KY030782.1).
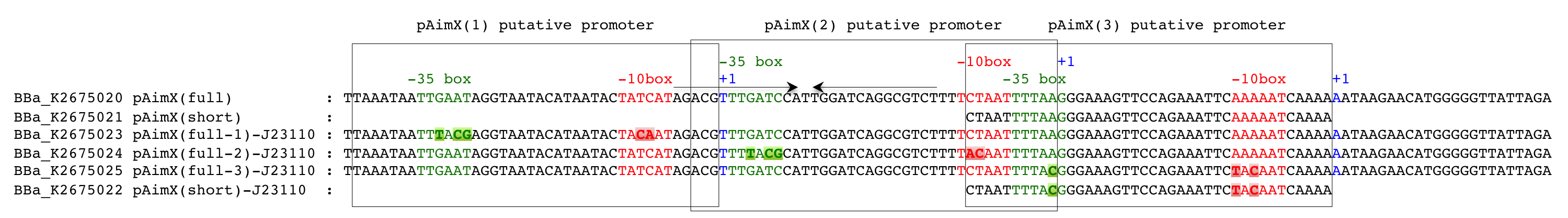
We have analysed the sequence of the pAimX(full) promoter using various available tools like the [http://www.fruitfly.org/seq_tools/promoter.html/ Neural Network Promoter Prediction web server], [http://linux1.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb BPROM websever] [2] or [http://rna.igmors.u-psud.fr/toolbox/arnold/index.php ARNold] [3, 4]. These sequence analyses revealed the presence of three putative promoters that we denote pAimX(1), pAimX(2) and pAimX(3), but also of an inverted repeat sequence potentially forming the stem-loop structure of a terminator.
To investigate the promoter activity of pAimX(full) sequence in E. coli, we have placed the reporter sfGFP with or without the LVAtag (BBa_K2675005 and BBa_K2675006) under its control and thus constructed the composite parts BBa_K2675050 and BBa_K2675060.
Considering the fact that the potential internal terminator overlaps mainly with the pAimX(2) putative promoter, we suspect that the AimX transcription could probably be initiated from the pAimX(3) putative promoter (BBa_K2675021). To investigate this possibility in E. coli, we also placed the reporter sfGFP with or without the LVAtag (BBa_K2675005 and BBa_K2675006) under the control of this short version of the pAimX promoter (pAimX(3) = pAimX(short) promoter) and thus constructed the BBa_K2675051 and BBa_K2675061.
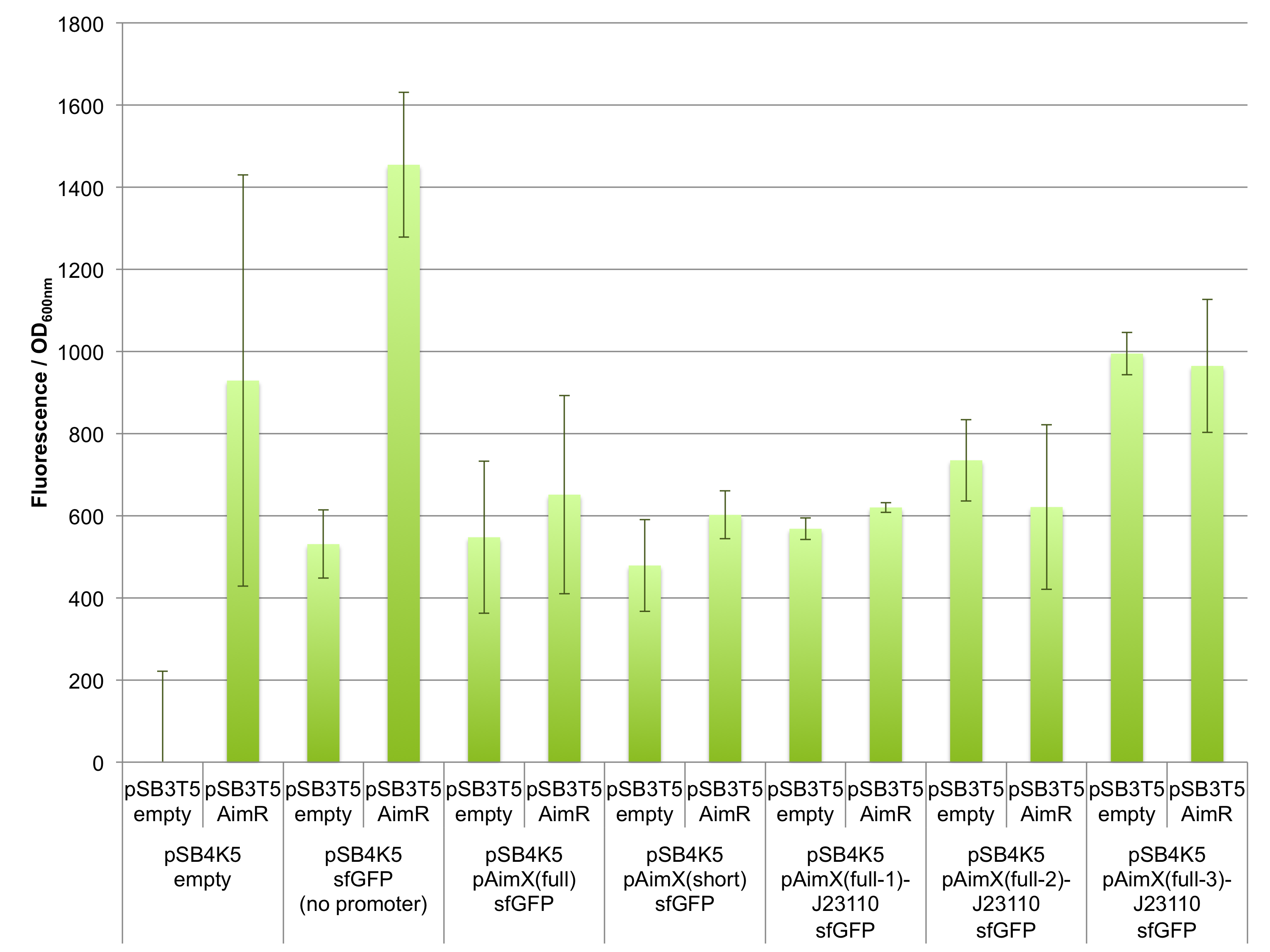
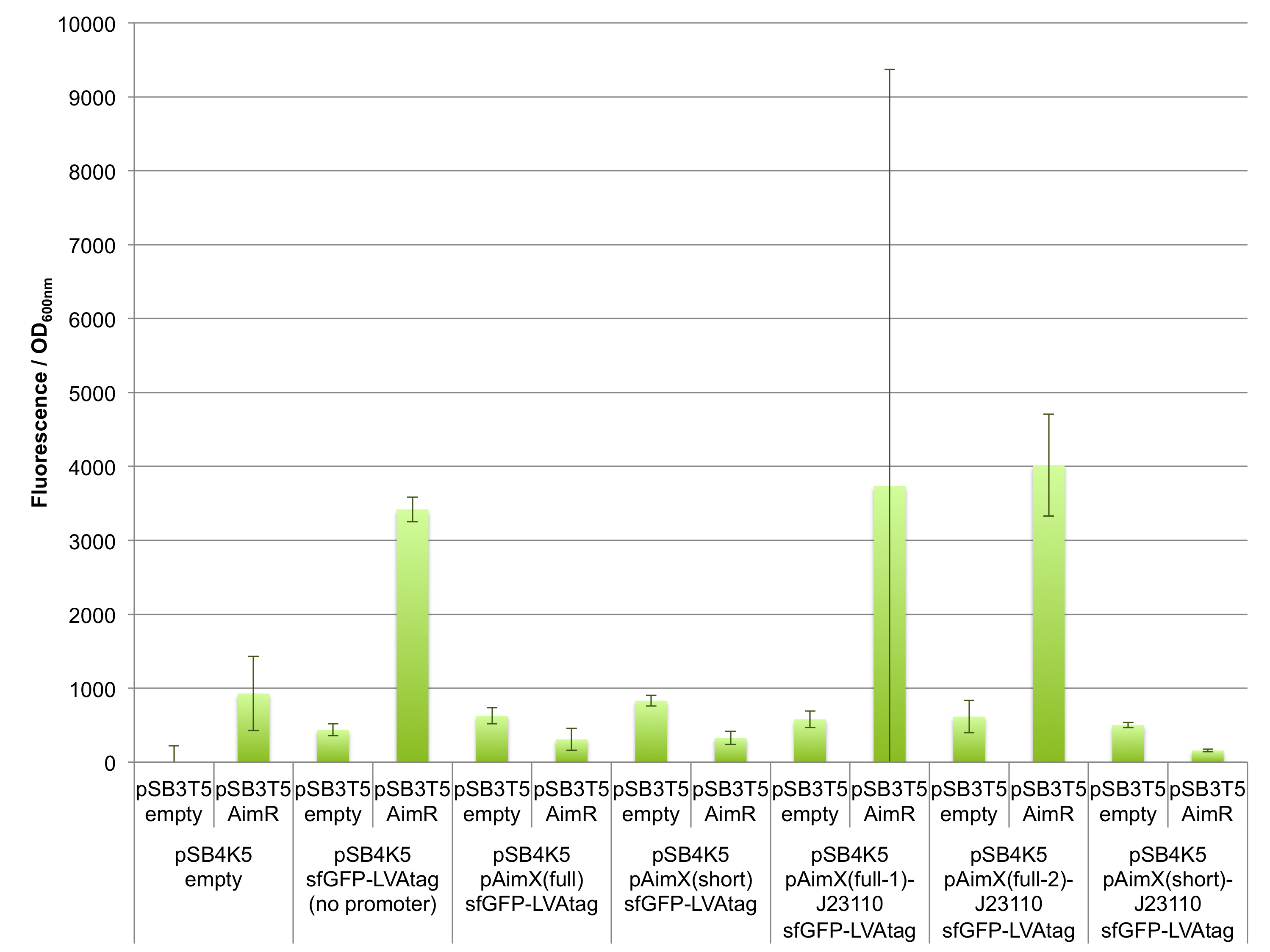
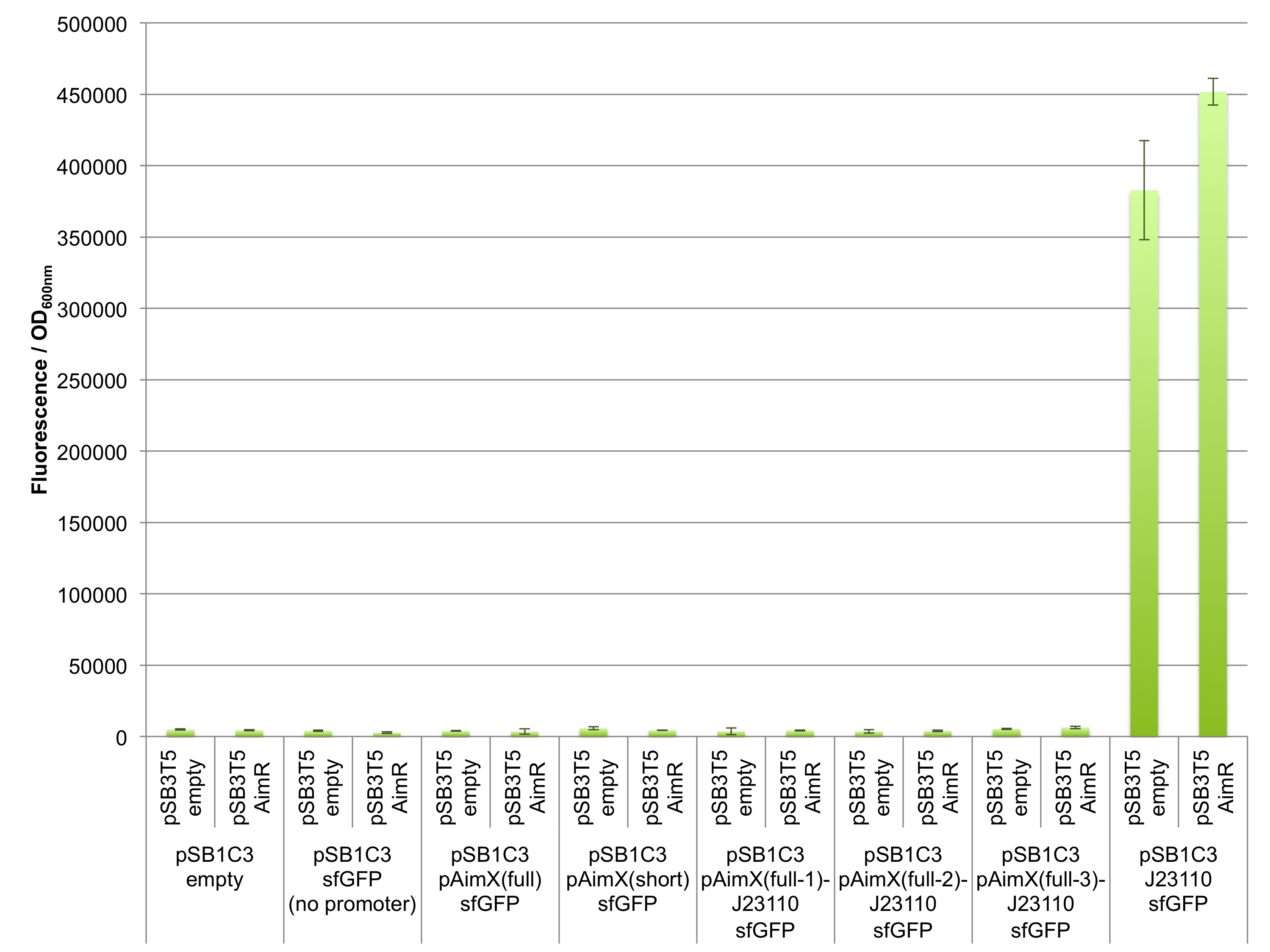
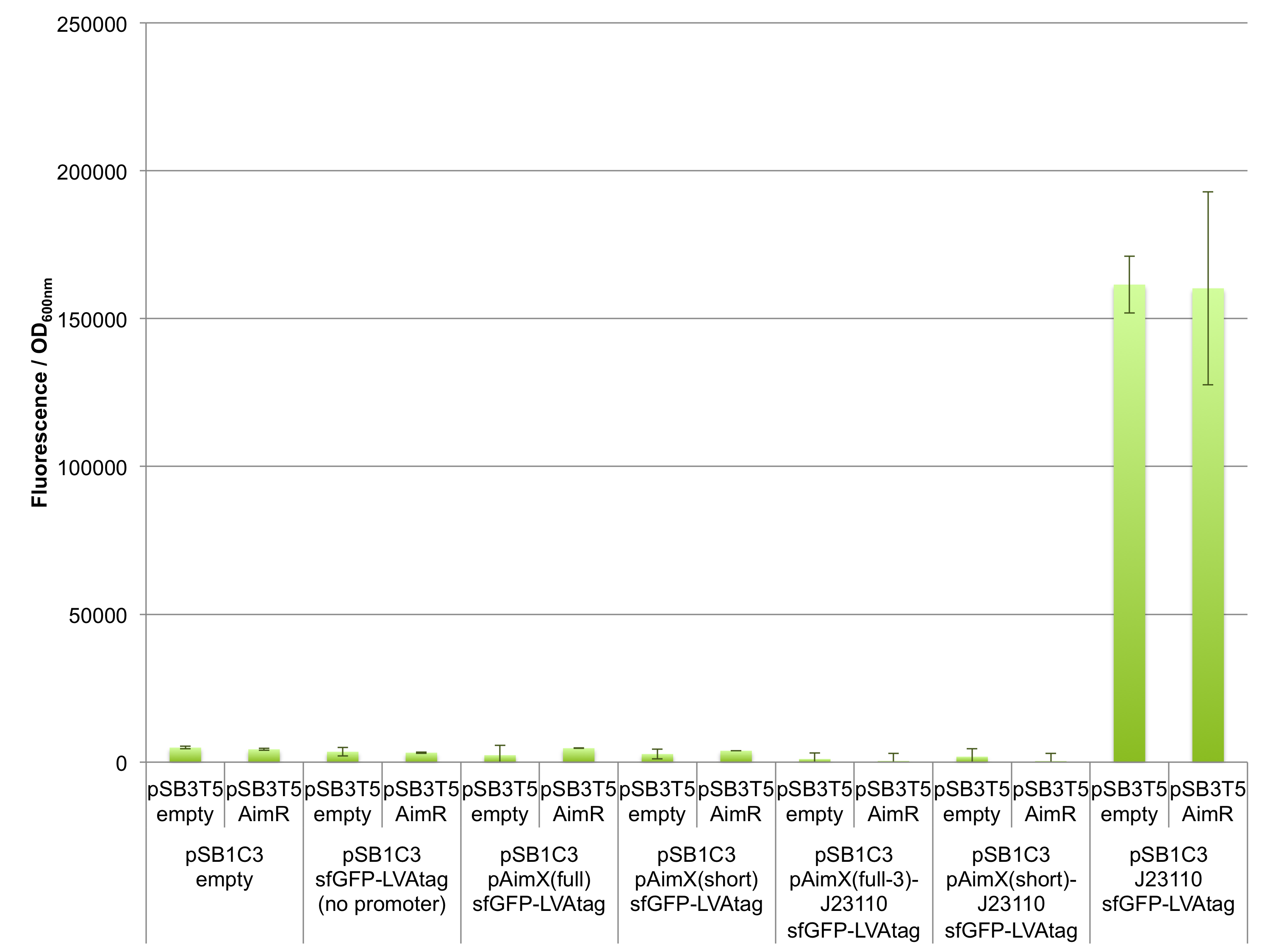
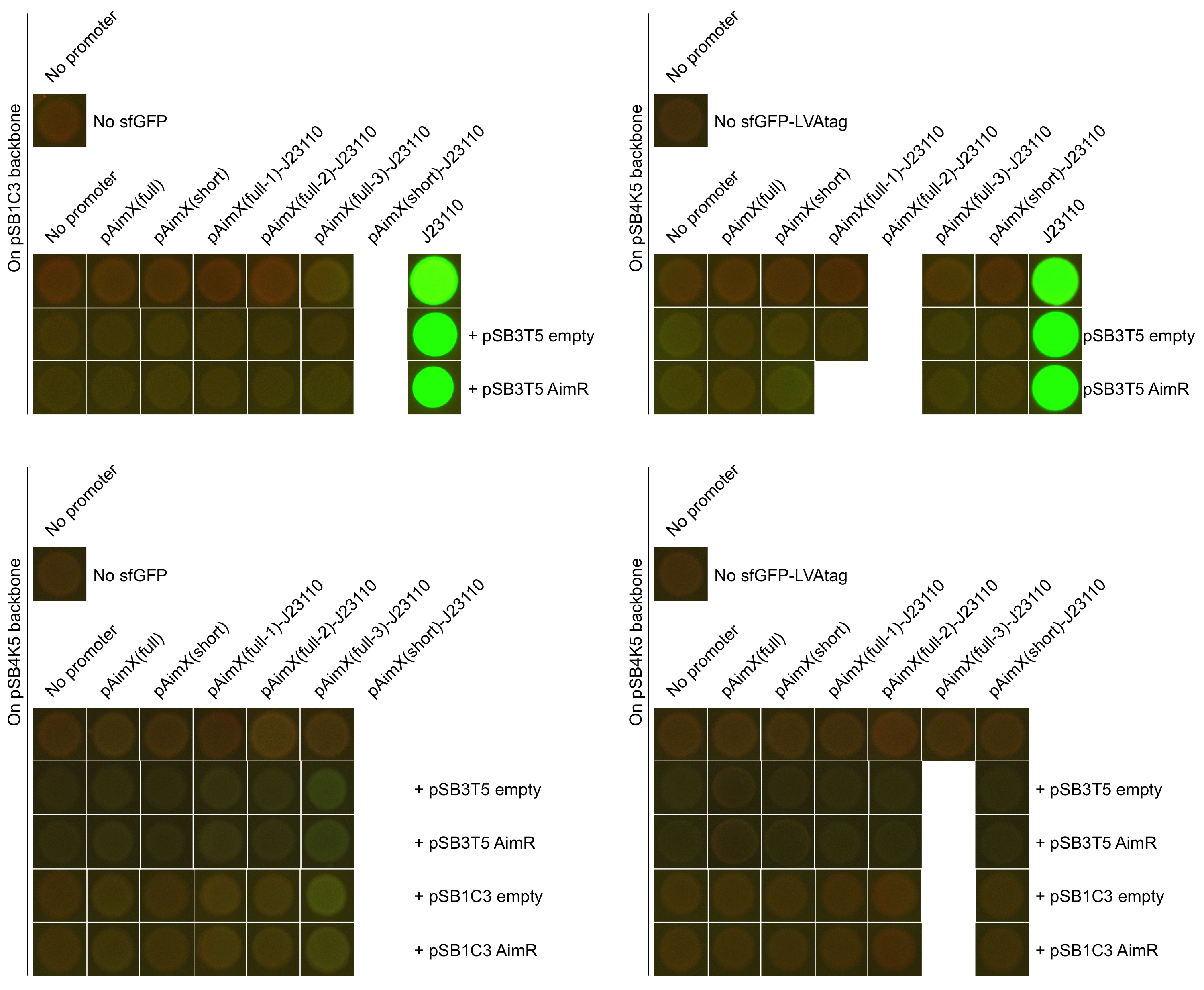
Fluorescence measurements of sfGFP in E. coli cells harbouring the 4 expression cassettes (BBa_K2675050, BBa_K2675051, BBa_K2675060 and BBa_K2675061) in both pSB1C3 and pSB4K5 backbones were unable to detect any significant sfGFP expression (Figure 2). This observation was visually confirmed when bacteria were spotted on plates (Figure 3). Even the presence of AimR (BBa_K2675040) did not have any effect on sfGFP expression from the pAimX(full) promoter (BBa_K2675020), nor the pAimX(short) promoter (BBa_K2675021) (Figures 1 and 2).
To improve the pAimx(full) and pAimX(short) promoter activity in E. coli, we decided to mutate the -35 and -10 boxes of these promoters and convert them to the ones of J23110 promoter (Figure 1). J23110 is a constitutive promoter from the Anderson Library with a medium transcription strength compared to the other members of this promoter family. In our system, using sfGFP or sfGFP-LVAtag (BBa_K2675056 and BBa_K2675066) as reporter, an intense fluorescence was detected (Figure 4).
Figure 1: pAimx(full)- and pAimX(short)-J23110 hybrid promoters. These mutated pAimX(full) and pAimx(short) promoters of phage phi3T AimX gene have modified -35 and -10 boxes. The mutated -35 and -10 boxes have the same sequences as BBa_J23110 promoter. -35 boxes are in green, -10 boxes in red and the transcription start sites in blue and indicated with +1. The inverted repeat sequences potentially forming the stem-loop structure of a terminator are marked by open horizontal arrows.
The results presented in Figures 2 and 3 show that the pAimx(full)- and pAimX(short)-J23110 hybrid promoters are not active in E. coli. Were were unable to detect any significant sfGFP expression regardless of the DNA copy number present in the cell (sfGFP on a high copy plasmid pSB1C3 or on a low copy plasmid pSB4K5), or of the presence of AimR expressed by BBa_K2675040 (from the high copy plasmid pSB1C3 or from the low copy plasmid pSB3T5).
Figure 2: In vivo characterization of sfGFP expression by E. coli cells harbouring the expression cassettes of sfGFP and sfGFP-LVAtag under the control of pAimX(full) promoter (BBa_K2675050 and BBa_K2675060), pAimX(short) promoter (BBa_K2675051 and BBa_K2675061), pAimx(full-1)-J23110 hybrid promoter (BBa_K2675053 and BBa_K2675063), pAimx(full-2)-J23110 hybrid promoter (BBa_K2675054 and BBa_K2675064), pAimx(full-3)-J23110 hybrid promoter (BBa_K2675055 and BBa_K2675065) and pAimX(short)-J23110 hybrid promoter (BBa_K2675052 and BBa_K2675062) in pSB1C3 or pSB4K5 backbones. The negative control has been performed with an empty pSB1C3 or pSB3K4 and the positive control with BBa_K2675056 and BBa_K2675066. The AimR was expressed as BBa_K2675040 on pSB3T5. Fluorescence values were normalised by OD600nm.
Figure 2: Pictures of E. coli cells harbouring the expression cassettes of sfGFP and sfGFP-LVAtag under the control of pAimX(full) promoter (BBa_K2675050 and BBa_K2675060), pAimX(short) promoter (BBa_K2675051 and BBa_K2675061), pAimx(full-1)-J23110 hybrid promoter (BBa_K2675053 and BBa_K2675063), pAimx(full-2)-J23110 hybrid promoter (BBa_K2675054 and BBa_K2675064), pAimx(full-3)-J23110 hybrid promoter (BBa_K2675055 and BBa_K2675065) and pAimX(short)-J23110 hybrid promoter (BBa_K2675052 and BBa_K2675062) in pSB1C3 or pSB4K5 backbones. The negative control has been performed with an empty pSB1C3 or pSB3K4 and the positive one with BBa_K2675056 and BBa_K2675066. In BBa_K2675056 and BBa_K2675066 sfGFP-LVAtag and sfGFP were equipped by a custom made RBS (BBa_K2675017) and placed under the control of the constitutive promoter (BBa_J23110). The AimR was expressed as BBa_K2675040 from the high copy plasmid pSB1C3 or the low copy plasmid pSB3T5. We can clearly see on the pictures a strong sfGFP-LVAtag and sfGFP expression by BBa_K2675056 and BBa_K2675066 and no significant expression by all other constructs.
From these data, we conclude that this part is not a functional expression cassette in E. coli.
References
[1] Erez Z, Steinberger-Levy I, Shamir M, Doron S, Stokar-Avihail A, Peleg Y, Melamed S, Leavitt A, Savidor A, Albeck S, Amitai G, Sorek R. Communication between viruses guides lysis-lysogeny decisions. Nature (2017) 541, 488-493.
[2] Solovyev V, Salamov A. Automatic Annotation of Microbial Genomes and Metagenomic Sequences. In Metagenomics and its Applications in Agriculture, Biomedicine and Environmental Studies (Ed. R.W. Li), Nova Science Publishers (2011) p. 61-78.
[3] Gautheret D, Lambert A. Direct RNA motif definition and identification from multiple sequence alignments using secondary structure profiles. J Mol Biol (2001) 313, 1003-1011.
[4] Macke T, Ecker D, Gutell R, Gautheret D, Case DA and Sampath R. RNAMotif – A new RNA secondary structure definition and discovery algorithm. Nucleic Acids Res (2001) 29, 4724–4735.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 187
- 1000COMPATIBLE WITH RFC[1000]
| None |